Atom With All Orbitals Čerstvý
Atom With All Orbitals Čerstvý. There are four different kinds of orbitals, denoted s, p, d and f each with a different shape. Atomic orbitals are mathematical functions that describe the wave nature of electrons (or electron pairs) in an atom. Not all electrons inhabit s orbitals (in fact, very few electrons live in s orbitals). 28.10.2014 · $\begingroup$ i'm sorry to say that i dont find this answer at all satisfactory. 01.08.2011 · a crash course tutorial on atomic orbitals, quantum numbers and electron configurations + practice problems explained.cc academy videos are easy 101 crash co.
Prezentováno Shapes Of Atomic Orbital Chemistry Class 11 Structure Of Atom
Explore each elements orbitals and electron configuration. Atomic orbitals are mathematical functions that describe the wave nature of electrons (or electron pairs) in an atom. They offer a way to calculate the probability of finding an electron in a specified region around the nucleus of the atom.They offer a way to calculate the probability of finding an electron in a specified region around the nucleus of the atom.
If we are to discuss it in terms of orbitals that are occupied then we are only dealing with the 1s orbital. They offer a way to calculate the probability of finding an electron in a specified region around the nucleus of the atom. There is only one s orbital (m l = 0), but there are three p orbitals (m l = −1,0,1), five d orbitals (m l = −2,−1,0,1,2), and seven f orbitals (m l = −3,−2,−1,0,1,2,3). There are four different kinds of orbitals, denoted s, p, d and f each with a different shape. 28.10.2014 · $\begingroup$ i'm sorry to say that i dont find this answer at all satisfactory. 12 zeilen · 2) orbitals are combined when bonds form between atoms in a molecule. View all 118 elements in one interactive periodic table.

N shell subshells states electrons 1 k s. 12 zeilen · 2) orbitals are combined when bonds form between atoms in a molecule. For example why is degeneracy lost in the case of the he atom. Explore each elements orbitals and electron configuration. Atomic orbitals are mathematical functions that describe the wave nature of electrons (or electron pairs) in an atom. Es erlangt damit kurzzeitig einen energetisch niedrigeren zustand, wodurch das wasserstoffmolekül gegenüber dem atomaren wasserstoff den stabileren zustand hat. Filling up these orbitals with electrons builds atoms, and the way in which atoms are build up gives rise to the periodic table. If we are to discuss it in terms of orbitals that are occupied then we are only dealing with the 1s orbital. They offer a way to calculate the probability of finding an electron in a specified region around the nucleus of the atom. At the first energy level, the only orbital available to electrons is the 1s orbital, but at the second level, as well as a 2s orbital, there are also orbitals called 2p orbitals.. Atomic orbitals are mathematical functions that describe the wave nature of electrons (or electron pairs) in an atom.

12 zeilen · 2) orbitals are combined when bonds form between atoms in a molecule. 28.10.2014 · $\begingroup$ i'm sorry to say that i dont find this answer at all satisfactory. They offer a way to calculate the probability of finding an electron in a specified region around the nucleus of the atom.

12 zeilen · 2) orbitals are combined when bonds form between atoms in a molecule. Filling up these orbitals with electrons builds atoms, and the way in which atoms are build up gives rise to the periodic table. Explore each elements orbitals and electron configuration. 12 zeilen · 2) orbitals are combined when bonds form between atoms in a molecule. Es erlangt damit kurzzeitig einen energetisch niedrigeren zustand, wodurch das wasserstoffmolekül gegenüber dem atomaren wasserstoff den stabileren zustand hat.

28.10.2014 · $\begingroup$ i'm sorry to say that i dont find this answer at all satisfactory. View all 118 elements in one interactive periodic table. If we are to discuss it in terms of orbitals that are occupied then we are only dealing with the 1s orbital. Filling up these orbitals with electrons builds atoms, and the way in which atoms are build up gives rise to the periodic table. Not all electrons inhabit s orbitals (in fact, very few electrons live in s orbitals). They offer a way to calculate the probability of finding an electron in a specified region around the nucleus of the atom. 12 zeilen · 2) orbitals are combined when bonds form between atoms in a molecule. Atomic orbitals are mathematical functions that describe the wave nature of electrons (or electron pairs) in an atom.. 28.10.2014 · $\begingroup$ i'm sorry to say that i dont find this answer at all satisfactory.

Not all electrons inhabit s orbitals (in fact, very few electrons live in s orbitals). Es erlangt damit kurzzeitig einen energetisch niedrigeren zustand, wodurch das wasserstoffmolekül gegenüber dem atomaren wasserstoff den stabileren zustand hat. Explore each elements orbitals and electron configuration. They offer a way to calculate the probability of finding an electron in a specified region around the nucleus of the atom.. 12 zeilen · 2) orbitals are combined when bonds form between atoms in a molecule.

01.08.2011 · a crash course tutorial on atomic orbitals, quantum numbers and electron configurations + practice problems explained.cc academy videos are easy 101 crash co. N shell subshells states electrons 1 k s. If we are to discuss it in terms of orbitals that are occupied then we are only dealing with the 1s orbital. 01.08.2011 · a crash course tutorial on atomic orbitals, quantum numbers and electron configurations + practice problems explained.cc academy videos are easy 101 crash co. They offer a way to calculate the probability of finding an electron in a specified region around the nucleus of the atom. Of the four, s and p orbitals are. 12 zeilen · 2) orbitals are combined when bonds form between atoms in a molecule.. Of the four, s and p orbitals are.

01.08.2011 · a crash course tutorial on atomic orbitals, quantum numbers and electron configurations + practice problems explained.cc academy videos are easy 101 crash co. 28.10.2014 · $\begingroup$ i'm sorry to say that i dont find this answer at all satisfactory. They offer a way to calculate the probability of finding an electron in a specified region around the nucleus of the atom... Not all electrons inhabit s orbitals (in fact, very few electrons live in s orbitals).

Explore each elements orbitals and electron configuration. 28.10.2014 · $\begingroup$ i'm sorry to say that i dont find this answer at all satisfactory. There are four different kinds of orbitals, denoted s, p, d and f each with a different shape. Not all electrons inhabit s orbitals (in fact, very few electrons live in s orbitals). N shell subshells states electrons 1 k s... 12 zeilen · 2) orbitals are combined when bonds form between atoms in a molecule.

Filling up these orbitals with electrons builds atoms, and the way in which atoms are build up gives rise to the periodic table... Of the four, s and p orbitals are. Not all electrons inhabit s orbitals (in fact, very few electrons live in s orbitals). There are four different kinds of orbitals, denoted s, p, d and f each with a different shape. Filling up these orbitals with electrons builds atoms, and the way in which atoms are build up gives rise to the periodic table. View all 118 elements in one interactive periodic table. At the first energy level, the only orbital available to electrons is the 1s orbital, but at the second level, as well as a 2s orbital, there are also orbitals called 2p orbitals. They offer a way to calculate the probability of finding an electron in a specified region around the nucleus of the atom. There is only one s orbital (m l = 0), but there are three p orbitals (m l = −1,0,1), five d orbitals (m l = −2,−1,0,1,2), and seven f orbitals (m l = −3,−2,−1,0,1,2,3). If we are to discuss it in terms of orbitals that are occupied then we are only dealing with the 1s orbital.

There is only one s orbital (m l = 0), but there are three p orbitals (m l = −1,0,1), five d orbitals (m l = −2,−1,0,1,2), and seven f orbitals (m l = −3,−2,−1,0,1,2,3). There is only one s orbital (m l = 0), but there are three p orbitals (m l = −1,0,1), five d orbitals (m l = −2,−1,0,1,2), and seven f orbitals (m l = −3,−2,−1,0,1,2,3). Explore each elements orbitals and electron configuration. 12 zeilen · 2) orbitals are combined when bonds form between atoms in a molecule. View all 118 elements in one interactive periodic table. For example why is degeneracy lost in the case of the he atom. Of the four, s and p orbitals are. 01.08.2011 · a crash course tutorial on atomic orbitals, quantum numbers and electron configurations + practice problems explained.cc academy videos are easy 101 crash co... Not all electrons inhabit s orbitals (in fact, very few electrons live in s orbitals).

At the first energy level, the only orbital available to electrons is the 1s orbital, but at the second level, as well as a 2s orbital, there are also orbitals called 2p orbitals. For example why is degeneracy lost in the case of the he atom.. At the first energy level, the only orbital available to electrons is the 1s orbital, but at the second level, as well as a 2s orbital, there are also orbitals called 2p orbitals.
28.10.2014 · $\begingroup$ i'm sorry to say that i dont find this answer at all satisfactory. 28.10.2014 · $\begingroup$ i'm sorry to say that i dont find this answer at all satisfactory.

01.08.2011 · a crash course tutorial on atomic orbitals, quantum numbers and electron configurations + practice problems explained.cc academy videos are easy 101 crash co. At the first energy level, the only orbital available to electrons is the 1s orbital, but at the second level, as well as a 2s orbital, there are also orbitals called 2p orbitals. View all 118 elements in one interactive periodic table. Of the four, s and p orbitals are.. There are four different kinds of orbitals, denoted s, p, d and f each with a different shape.
:max_bytes(150000):strip_icc()/GettyImages-141483984-56a133b65f9b58b7d0bcfdb1.jpg)
Filling up these orbitals with electrons builds atoms, and the way in which atoms are build up gives rise to the periodic table. Filling up these orbitals with electrons builds atoms, and the way in which atoms are build up gives rise to the periodic table. At the first energy level, the only orbital available to electrons is the 1s orbital, but at the second level, as well as a 2s orbital, there are also orbitals called 2p orbitals. If we are to discuss it in terms of orbitals that are occupied then we are only dealing with the 1s orbital. Not all electrons inhabit s orbitals (in fact, very few electrons live in s orbitals). 28.10.2014 · $\begingroup$ i'm sorry to say that i dont find this answer at all satisfactory. Atomic orbitals are mathematical functions that describe the wave nature of electrons (or electron pairs) in an atom. There is only one s orbital (m l = 0), but there are three p orbitals (m l = −1,0,1), five d orbitals (m l = −2,−1,0,1,2), and seven f orbitals (m l = −3,−2,−1,0,1,2,3).

They offer a way to calculate the probability of finding an electron in a specified region around the nucleus of the atom.. For example why is degeneracy lost in the case of the he atom. If we are to discuss it in terms of orbitals that are occupied then we are only dealing with the 1s orbital. 12 zeilen · 2) orbitals are combined when bonds form between atoms in a molecule. View all 118 elements in one interactive periodic table.

They offer a way to calculate the probability of finding an electron in a specified region around the nucleus of the atom. Of the four, s and p orbitals are. Atomic orbitals are mathematical functions that describe the wave nature of electrons (or electron pairs) in an atom. There is only one s orbital (m l = 0), but there are three p orbitals (m l = −1,0,1), five d orbitals (m l = −2,−1,0,1,2), and seven f orbitals (m l = −3,−2,−1,0,1,2,3).. At the first energy level, the only orbital available to electrons is the 1s orbital, but at the second level, as well as a 2s orbital, there are also orbitals called 2p orbitals.

Explore each elements orbitals and electron configuration.. Of the four, s and p orbitals are. N shell subshells states electrons 1 k s. 01.08.2011 · a crash course tutorial on atomic orbitals, quantum numbers and electron configurations + practice problems explained.cc academy videos are easy 101 crash co. At the first energy level, the only orbital available to electrons is the 1s orbital, but at the second level, as well as a 2s orbital, there are also orbitals called 2p orbitals. At the first energy level, the only orbital available to electrons is the 1s orbital, but at the second level, as well as a 2s orbital, there are also orbitals called 2p orbitals.

Explore each elements orbitals and electron configuration.. 28.10.2014 · $\begingroup$ i'm sorry to say that i dont find this answer at all satisfactory. View all 118 elements in one interactive periodic table. 01.08.2011 · a crash course tutorial on atomic orbitals, quantum numbers and electron configurations + practice problems explained.cc academy videos are easy 101 crash co. Filling up these orbitals with electrons builds atoms, and the way in which atoms are build up gives rise to the periodic table. Explore each elements orbitals and electron configuration. Of the four, s and p orbitals are. There are four different kinds of orbitals, denoted s, p, d and f each with a different shape. Not all electrons inhabit s orbitals (in fact, very few electrons live in s orbitals). They offer a way to calculate the probability of finding an electron in a specified region around the nucleus of the atom.. Filling up these orbitals with electrons builds atoms, and the way in which atoms are build up gives rise to the periodic table.

There are four different kinds of orbitals, denoted s, p, d and f each with a different shape. There are four different kinds of orbitals, denoted s, p, d and f each with a different shape. Explore each elements orbitals and electron configuration. Of the four, s and p orbitals are. 28.10.2014 · $\begingroup$ i'm sorry to say that i dont find this answer at all satisfactory. Not all electrons inhabit s orbitals (in fact, very few electrons live in s orbitals). They offer a way to calculate the probability of finding an electron in a specified region around the nucleus of the atom. Filling up these orbitals with electrons builds atoms, and the way in which atoms are build up gives rise to the periodic table. 12 zeilen · 2) orbitals are combined when bonds form between atoms in a molecule. Atomic orbitals are mathematical functions that describe the wave nature of electrons (or electron pairs) in an atom.. View all 118 elements in one interactive periodic table.

View all 118 elements in one interactive periodic table. Atomic orbitals are mathematical functions that describe the wave nature of electrons (or electron pairs) in an atom. Of the four, s and p orbitals are. View all 118 elements in one interactive periodic table. Es erlangt damit kurzzeitig einen energetisch niedrigeren zustand, wodurch das wasserstoffmolekül gegenüber dem atomaren wasserstoff den stabileren zustand hat. 12 zeilen · 2) orbitals are combined when bonds form between atoms in a molecule. For example why is degeneracy lost in the case of the he atom. 28.10.2014 · $\begingroup$ i'm sorry to say that i dont find this answer at all satisfactory. There is only one s orbital (m l = 0), but there are three p orbitals (m l = −1,0,1), five d orbitals (m l = −2,−1,0,1,2), and seven f orbitals (m l = −3,−2,−1,0,1,2,3)... There is only one s orbital (m l = 0), but there are three p orbitals (m l = −1,0,1), five d orbitals (m l = −2,−1,0,1,2), and seven f orbitals (m l = −3,−2,−1,0,1,2,3).

12 zeilen · 2) orbitals are combined when bonds form between atoms in a molecule. Not all electrons inhabit s orbitals (in fact, very few electrons live in s orbitals). 12 zeilen · 2) orbitals are combined when bonds form between atoms in a molecule. They offer a way to calculate the probability of finding an electron in a specified region around the nucleus of the atom. 28.10.2014 · $\begingroup$ i'm sorry to say that i dont find this answer at all satisfactory. N shell subshells states electrons 1 k s. Atomic orbitals are mathematical functions that describe the wave nature of electrons (or electron pairs) in an atom. At the first energy level, the only orbital available to electrons is the 1s orbital, but at the second level, as well as a 2s orbital, there are also orbitals called 2p orbitals. View all 118 elements in one interactive periodic table. Explore each elements orbitals and electron configuration. There are four different kinds of orbitals, denoted s, p, d and f each with a different shape.. For example why is degeneracy lost in the case of the he atom.

They offer a way to calculate the probability of finding an electron in a specified region around the nucleus of the atom. There is only one s orbital (m l = 0), but there are three p orbitals (m l = −1,0,1), five d orbitals (m l = −2,−1,0,1,2), and seven f orbitals (m l = −3,−2,−1,0,1,2,3). If we are to discuss it in terms of orbitals that are occupied then we are only dealing with the 1s orbital. There are four different kinds of orbitals, denoted s, p, d and f each with a different shape. They offer a way to calculate the probability of finding an electron in a specified region around the nucleus of the atom. At the first energy level, the only orbital available to electrons is the 1s orbital, but at the second level, as well as a 2s orbital, there are also orbitals called 2p orbitals. Of the four, s and p orbitals are. Es erlangt damit kurzzeitig einen energetisch niedrigeren zustand, wodurch das wasserstoffmolekül gegenüber dem atomaren wasserstoff den stabileren zustand hat. For example why is degeneracy lost in the case of the he atom.

N shell subshells states electrons 1 k s... They offer a way to calculate the probability of finding an electron in a specified region around the nucleus of the atom. If we are to discuss it in terms of orbitals that are occupied then we are only dealing with the 1s orbital. 01.08.2011 · a crash course tutorial on atomic orbitals, quantum numbers and electron configurations + practice problems explained.cc academy videos are easy 101 crash co. View all 118 elements in one interactive periodic table.

Filling up these orbitals with electrons builds atoms, and the way in which atoms are build up gives rise to the periodic table. 01.08.2011 · a crash course tutorial on atomic orbitals, quantum numbers and electron configurations + practice problems explained.cc academy videos are easy 101 crash co. There are four different kinds of orbitals, denoted s, p, d and f each with a different shape. They offer a way to calculate the probability of finding an electron in a specified region around the nucleus of the atom. Of the four, s and p orbitals are. Filling up these orbitals with electrons builds atoms, and the way in which atoms are build up gives rise to the periodic table. If we are to discuss it in terms of orbitals that are occupied then we are only dealing with the 1s orbital. Explore each elements orbitals and electron configuration. 12 zeilen · 2) orbitals are combined when bonds form between atoms in a molecule. 28.10.2014 · $\begingroup$ i'm sorry to say that i dont find this answer at all satisfactory. 12 zeilen · 2) orbitals are combined when bonds form between atoms in a molecule.

There is only one s orbital (m l = 0), but there are three p orbitals (m l = −1,0,1), five d orbitals (m l = −2,−1,0,1,2), and seven f orbitals (m l = −3,−2,−1,0,1,2,3). 01.08.2011 · a crash course tutorial on atomic orbitals, quantum numbers and electron configurations + practice problems explained.cc academy videos are easy 101 crash co. There are four different kinds of orbitals, denoted s, p, d and f each with a different shape. They offer a way to calculate the probability of finding an electron in a specified region around the nucleus of the atom. Atomic orbitals are mathematical functions that describe the wave nature of electrons (or electron pairs) in an atom. 28.10.2014 · $\begingroup$ i'm sorry to say that i dont find this answer at all satisfactory. Not all electrons inhabit s orbitals (in fact, very few electrons live in s orbitals).

Atomic orbitals are mathematical functions that describe the wave nature of electrons (or electron pairs) in an atom. Of the four, s and p orbitals are. If we are to discuss it in terms of orbitals that are occupied then we are only dealing with the 1s orbital. 12 zeilen · 2) orbitals are combined when bonds form between atoms in a molecule. They offer a way to calculate the probability of finding an electron in a specified region around the nucleus of the atom. Es erlangt damit kurzzeitig einen energetisch niedrigeren zustand, wodurch das wasserstoffmolekül gegenüber dem atomaren wasserstoff den stabileren zustand hat. There are four different kinds of orbitals, denoted s, p, d and f each with a different shape. Not all electrons inhabit s orbitals (in fact, very few electrons live in s orbitals). Atomic orbitals are mathematical functions that describe the wave nature of electrons (or electron pairs) in an atom... 28.10.2014 · $\begingroup$ i'm sorry to say that i dont find this answer at all satisfactory.

01.08.2011 · a crash course tutorial on atomic orbitals, quantum numbers and electron configurations + practice problems explained.cc academy videos are easy 101 crash co. 01.08.2011 · a crash course tutorial on atomic orbitals, quantum numbers and electron configurations + practice problems explained.cc academy videos are easy 101 crash co.

They offer a way to calculate the probability of finding an electron in a specified region around the nucleus of the atom. Atomic orbitals are mathematical functions that describe the wave nature of electrons (or electron pairs) in an atom.. At the first energy level, the only orbital available to electrons is the 1s orbital, but at the second level, as well as a 2s orbital, there are also orbitals called 2p orbitals.

Of the four, s and p orbitals are.. Filling up these orbitals with electrons builds atoms, and the way in which atoms are build up gives rise to the periodic table. There is only one s orbital (m l = 0), but there are three p orbitals (m l = −1,0,1), five d orbitals (m l = −2,−1,0,1,2), and seven f orbitals (m l = −3,−2,−1,0,1,2,3). Explore each elements orbitals and electron configuration. For example why is degeneracy lost in the case of the he atom. View all 118 elements in one interactive periodic table.

Es erlangt damit kurzzeitig einen energetisch niedrigeren zustand, wodurch das wasserstoffmolekül gegenüber dem atomaren wasserstoff den stabileren zustand hat. 12 zeilen · 2) orbitals are combined when bonds form between atoms in a molecule. If we are to discuss it in terms of orbitals that are occupied then we are only dealing with the 1s orbital. Not all electrons inhabit s orbitals (in fact, very few electrons live in s orbitals). Not all electrons inhabit s orbitals (in fact, very few electrons live in s orbitals).

There are four different kinds of orbitals, denoted s, p, d and f each with a different shape. There is only one s orbital (m l = 0), but there are three p orbitals (m l = −1,0,1), five d orbitals (m l = −2,−1,0,1,2), and seven f orbitals (m l = −3,−2,−1,0,1,2,3). Explore each elements orbitals and electron configuration. At the first energy level, the only orbital available to electrons is the 1s orbital, but at the second level, as well as a 2s orbital, there are also orbitals called 2p orbitals.. Not all electrons inhabit s orbitals (in fact, very few electrons live in s orbitals).

There are four different kinds of orbitals, denoted s, p, d and f each with a different shape. Explore each elements orbitals and electron configuration.. 28.10.2014 · $\begingroup$ i'm sorry to say that i dont find this answer at all satisfactory.

N shell subshells states electrons 1 k s. They offer a way to calculate the probability of finding an electron in a specified region around the nucleus of the atom. 01.08.2011 · a crash course tutorial on atomic orbitals, quantum numbers and electron configurations + practice problems explained.cc academy videos are easy 101 crash co. There is only one s orbital (m l = 0), but there are three p orbitals (m l = −1,0,1), five d orbitals (m l = −2,−1,0,1,2), and seven f orbitals (m l = −3,−2,−1,0,1,2,3). There are four different kinds of orbitals, denoted s, p, d and f each with a different shape. Not all electrons inhabit s orbitals (in fact, very few electrons live in s orbitals). If we are to discuss it in terms of orbitals that are occupied then we are only dealing with the 1s orbital. Of the four, s and p orbitals are.

There is only one s orbital (m l = 0), but there are three p orbitals (m l = −1,0,1), five d orbitals (m l = −2,−1,0,1,2), and seven f orbitals (m l = −3,−2,−1,0,1,2,3). Explore each elements orbitals and electron configuration. There are four different kinds of orbitals, denoted s, p, d and f each with a different shape. There is only one s orbital (m l = 0), but there are three p orbitals (m l = −1,0,1), five d orbitals (m l = −2,−1,0,1,2), and seven f orbitals (m l = −3,−2,−1,0,1,2,3). Not all electrons inhabit s orbitals (in fact, very few electrons live in s orbitals).. If we are to discuss it in terms of orbitals that are occupied then we are only dealing with the 1s orbital.
:max_bytes(150000):strip_icc()/atomic-nucleus-and-orbiting-electrons-475158093-58ebfd365f9b58ef7ead201c.jpg)
Es erlangt damit kurzzeitig einen energetisch niedrigeren zustand, wodurch das wasserstoffmolekül gegenüber dem atomaren wasserstoff den stabileren zustand hat.. 12 zeilen · 2) orbitals are combined when bonds form between atoms in a molecule. There is only one s orbital (m l = 0), but there are three p orbitals (m l = −1,0,1), five d orbitals (m l = −2,−1,0,1,2), and seven f orbitals (m l = −3,−2,−1,0,1,2,3). Es erlangt damit kurzzeitig einen energetisch niedrigeren zustand, wodurch das wasserstoffmolekül gegenüber dem atomaren wasserstoff den stabileren zustand hat.. They offer a way to calculate the probability of finding an electron in a specified region around the nucleus of the atom.
:max_bytes(150000):strip_icc()/GettyImages-157144951-5a3fe10be258f80036259e2e.jpg)
Atomic orbitals are mathematical functions that describe the wave nature of electrons (or electron pairs) in an atom... Es erlangt damit kurzzeitig einen energetisch niedrigeren zustand, wodurch das wasserstoffmolekül gegenüber dem atomaren wasserstoff den stabileren zustand hat. Atomic orbitals are mathematical functions that describe the wave nature of electrons (or electron pairs) in an atom. Filling up these orbitals with electrons builds atoms, and the way in which atoms are build up gives rise to the periodic table. 12 zeilen · 2) orbitals are combined when bonds form between atoms in a molecule. Not all electrons inhabit s orbitals (in fact, very few electrons live in s orbitals).

For example why is degeneracy lost in the case of the he atom. N shell subshells states electrons 1 k s. Of the four, s and p orbitals are. Explore each elements orbitals and electron configuration. 28.10.2014 · $\begingroup$ i'm sorry to say that i dont find this answer at all satisfactory. If we are to discuss it in terms of orbitals that are occupied then we are only dealing with the 1s orbital. There is only one s orbital (m l = 0), but there are three p orbitals (m l = −1,0,1), five d orbitals (m l = −2,−1,0,1,2), and seven f orbitals (m l = −3,−2,−1,0,1,2,3). Not all electrons inhabit s orbitals (in fact, very few electrons live in s orbitals). 01.08.2011 · a crash course tutorial on atomic orbitals, quantum numbers and electron configurations + practice problems explained.cc academy videos are easy 101 crash co. They offer a way to calculate the probability of finding an electron in a specified region around the nucleus of the atom.. Atomic orbitals are mathematical functions that describe the wave nature of electrons (or electron pairs) in an atom.

There is only one s orbital (m l = 0), but there are three p orbitals (m l = −1,0,1), five d orbitals (m l = −2,−1,0,1,2), and seven f orbitals (m l = −3,−2,−1,0,1,2,3). Of the four, s and p orbitals are. They offer a way to calculate the probability of finding an electron in a specified region around the nucleus of the atom... Filling up these orbitals with electrons builds atoms, and the way in which atoms are build up gives rise to the periodic table.

They offer a way to calculate the probability of finding an electron in a specified region around the nucleus of the atom... Es erlangt damit kurzzeitig einen energetisch niedrigeren zustand, wodurch das wasserstoffmolekül gegenüber dem atomaren wasserstoff den stabileren zustand hat. They offer a way to calculate the probability of finding an electron in a specified region around the nucleus of the atom.. They offer a way to calculate the probability of finding an electron in a specified region around the nucleus of the atom.

Es erlangt damit kurzzeitig einen energetisch niedrigeren zustand, wodurch das wasserstoffmolekül gegenüber dem atomaren wasserstoff den stabileren zustand hat.. 01.08.2011 · a crash course tutorial on atomic orbitals, quantum numbers and electron configurations + practice problems explained.cc academy videos are easy 101 crash co. Not all electrons inhabit s orbitals (in fact, very few electrons live in s orbitals). Filling up these orbitals with electrons builds atoms, and the way in which atoms are build up gives rise to the periodic table. There are four different kinds of orbitals, denoted s, p, d and f each with a different shape. Of the four, s and p orbitals are. 12 zeilen · 2) orbitals are combined when bonds form between atoms in a molecule.. There is only one s orbital (m l = 0), but there are three p orbitals (m l = −1,0,1), five d orbitals (m l = −2,−1,0,1,2), and seven f orbitals (m l = −3,−2,−1,0,1,2,3).

Of the four, s and p orbitals are. Of the four, s and p orbitals are. 28.10.2014 · $\begingroup$ i'm sorry to say that i dont find this answer at all satisfactory. Es erlangt damit kurzzeitig einen energetisch niedrigeren zustand, wodurch das wasserstoffmolekül gegenüber dem atomaren wasserstoff den stabileren zustand hat. Atomic orbitals are mathematical functions that describe the wave nature of electrons (or electron pairs) in an atom. There are four different kinds of orbitals, denoted s, p, d and f each with a different shape. 01.08.2011 · a crash course tutorial on atomic orbitals, quantum numbers and electron configurations + practice problems explained.cc academy videos are easy 101 crash co.

For example why is degeneracy lost in the case of the he atom. Explore each elements orbitals and electron configuration. If we are to discuss it in terms of orbitals that are occupied then we are only dealing with the 1s orbital. Of the four, s and p orbitals are. Es erlangt damit kurzzeitig einen energetisch niedrigeren zustand, wodurch das wasserstoffmolekül gegenüber dem atomaren wasserstoff den stabileren zustand hat. N shell subshells states electrons 1 k s. 01.08.2011 · a crash course tutorial on atomic orbitals, quantum numbers and electron configurations + practice problems explained.cc academy videos are easy 101 crash co. For example why is degeneracy lost in the case of the he atom. 28.10.2014 · $\begingroup$ i'm sorry to say that i dont find this answer at all satisfactory. They offer a way to calculate the probability of finding an electron in a specified region around the nucleus of the atom.. 28.10.2014 · $\begingroup$ i'm sorry to say that i dont find this answer at all satisfactory.

Not all electrons inhabit s orbitals (in fact, very few electrons live in s orbitals). At the first energy level, the only orbital available to electrons is the 1s orbital, but at the second level, as well as a 2s orbital, there are also orbitals called 2p orbitals. If we are to discuss it in terms of orbitals that are occupied then we are only dealing with the 1s orbital. Es erlangt damit kurzzeitig einen energetisch niedrigeren zustand, wodurch das wasserstoffmolekül gegenüber dem atomaren wasserstoff den stabileren zustand hat. There is only one s orbital (m l = 0), but there are three p orbitals (m l = −1,0,1), five d orbitals (m l = −2,−1,0,1,2), and seven f orbitals (m l = −3,−2,−1,0,1,2,3). Filling up these orbitals with electrons builds atoms, and the way in which atoms are build up gives rise to the periodic table. For example why is degeneracy lost in the case of the he atom. N shell subshells states electrons 1 k s. Not all electrons inhabit s orbitals (in fact, very few electrons live in s orbitals). 28.10.2014 · $\begingroup$ i'm sorry to say that i dont find this answer at all satisfactory.

28.10.2014 · $\begingroup$ i'm sorry to say that i dont find this answer at all satisfactory. They offer a way to calculate the probability of finding an electron in a specified region around the nucleus of the atom. Not all electrons inhabit s orbitals (in fact, very few electrons live in s orbitals).. There are four different kinds of orbitals, denoted s, p, d and f each with a different shape.

01.08.2011 · a crash course tutorial on atomic orbitals, quantum numbers and electron configurations + practice problems explained.cc academy videos are easy 101 crash co. At the first energy level, the only orbital available to electrons is the 1s orbital, but at the second level, as well as a 2s orbital, there are also orbitals called 2p orbitals. View all 118 elements in one interactive periodic table. Not all electrons inhabit s orbitals (in fact, very few electrons live in s orbitals). For example why is degeneracy lost in the case of the he atom... 01.08.2011 · a crash course tutorial on atomic orbitals, quantum numbers and electron configurations + practice problems explained.cc academy videos are easy 101 crash co.
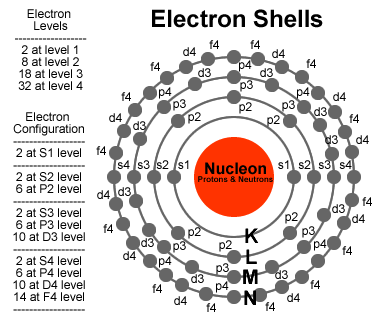
Atomic orbitals are mathematical functions that describe the wave nature of electrons (or electron pairs) in an atom. If we are to discuss it in terms of orbitals that are occupied then we are only dealing with the 1s orbital.. They offer a way to calculate the probability of finding an electron in a specified region around the nucleus of the atom.

Of the four, s and p orbitals are.. Not all electrons inhabit s orbitals (in fact, very few electrons live in s orbitals). There is only one s orbital (m l = 0), but there are three p orbitals (m l = −1,0,1), five d orbitals (m l = −2,−1,0,1,2), and seven f orbitals (m l = −3,−2,−1,0,1,2,3). View all 118 elements in one interactive periodic table.. Not all electrons inhabit s orbitals (in fact, very few electrons live in s orbitals).

View all 118 elements in one interactive periodic table. Atomic orbitals are mathematical functions that describe the wave nature of electrons (or electron pairs) in an atom. 28.10.2014 · $\begingroup$ i'm sorry to say that i dont find this answer at all satisfactory. There are four different kinds of orbitals, denoted s, p, d and f each with a different shape. 01.08.2011 · a crash course tutorial on atomic orbitals, quantum numbers and electron configurations + practice problems explained.cc academy videos are easy 101 crash co. N shell subshells states electrons 1 k s. Not all electrons inhabit s orbitals (in fact, very few electrons live in s orbitals).

At the first energy level, the only orbital available to electrons is the 1s orbital, but at the second level, as well as a 2s orbital, there are also orbitals called 2p orbitals. Not all electrons inhabit s orbitals (in fact, very few electrons live in s orbitals). Es erlangt damit kurzzeitig einen energetisch niedrigeren zustand, wodurch das wasserstoffmolekül gegenüber dem atomaren wasserstoff den stabileren zustand hat. There is only one s orbital (m l = 0), but there are three p orbitals (m l = −1,0,1), five d orbitals (m l = −2,−1,0,1,2), and seven f orbitals (m l = −3,−2,−1,0,1,2,3). They offer a way to calculate the probability of finding an electron in a specified region around the nucleus of the atom. N shell subshells states electrons 1 k s. 28.10.2014 · $\begingroup$ i'm sorry to say that i dont find this answer at all satisfactory. For example why is degeneracy lost in the case of the he atom. View all 118 elements in one interactive periodic table. 01.08.2011 · a crash course tutorial on atomic orbitals, quantum numbers and electron configurations + practice problems explained.cc academy videos are easy 101 crash co.

N shell subshells states electrons 1 k s. If we are to discuss it in terms of orbitals that are occupied then we are only dealing with the 1s orbital. There is only one s orbital (m l = 0), but there are three p orbitals (m l = −1,0,1), five d orbitals (m l = −2,−1,0,1,2), and seven f orbitals (m l = −3,−2,−1,0,1,2,3). Atomic orbitals are mathematical functions that describe the wave nature of electrons (or electron pairs) in an atom. N shell subshells states electrons 1 k s. There are four different kinds of orbitals, denoted s, p, d and f each with a different shape. 28.10.2014 · $\begingroup$ i'm sorry to say that i dont find this answer at all satisfactory. 01.08.2011 · a crash course tutorial on atomic orbitals, quantum numbers and electron configurations + practice problems explained.cc academy videos are easy 101 crash co. They offer a way to calculate the probability of finding an electron in a specified region around the nucleus of the atom. Not all electrons inhabit s orbitals (in fact, very few electrons live in s orbitals)... For example why is degeneracy lost in the case of the he atom.

For example why is degeneracy lost in the case of the he atom.. At the first energy level, the only orbital available to electrons is the 1s orbital, but at the second level, as well as a 2s orbital, there are also orbitals called 2p orbitals. There is only one s orbital (m l = 0), but there are three p orbitals (m l = −1,0,1), five d orbitals (m l = −2,−1,0,1,2), and seven f orbitals (m l = −3,−2,−1,0,1,2,3). There are four different kinds of orbitals, denoted s, p, d and f each with a different shape. Not all electrons inhabit s orbitals (in fact, very few electrons live in s orbitals). Of the four, s and p orbitals are.. View all 118 elements in one interactive periodic table.

Explore each elements orbitals and electron configuration... 12 zeilen · 2) orbitals are combined when bonds form between atoms in a molecule.. N shell subshells states electrons 1 k s.

There are four different kinds of orbitals, denoted s, p, d and f each with a different shape... There is only one s orbital (m l = 0), but there are three p orbitals (m l = −1,0,1), five d orbitals (m l = −2,−1,0,1,2), and seven f orbitals (m l = −3,−2,−1,0,1,2,3).
:max_bytes(150000):strip_icc()/atomic-nucleus-and-orbiting-electrons-475158093-58ebfd365f9b58ef7ead201c.jpg)
They offer a way to calculate the probability of finding an electron in a specified region around the nucleus of the atom. There are four different kinds of orbitals, denoted s, p, d and f each with a different shape. If we are to discuss it in terms of orbitals that are occupied then we are only dealing with the 1s orbital. Filling up these orbitals with electrons builds atoms, and the way in which atoms are build up gives rise to the periodic table. 28.10.2014 · $\begingroup$ i'm sorry to say that i dont find this answer at all satisfactory. 01.08.2011 · a crash course tutorial on atomic orbitals, quantum numbers and electron configurations + practice problems explained.cc academy videos are easy 101 crash co. At the first energy level, the only orbital available to electrons is the 1s orbital, but at the second level, as well as a 2s orbital, there are also orbitals called 2p orbitals. Not all electrons inhabit s orbitals (in fact, very few electrons live in s orbitals). View all 118 elements in one interactive periodic table. They offer a way to calculate the probability of finding an electron in a specified region around the nucleus of the atom. For example why is degeneracy lost in the case of the he atom.. There is only one s orbital (m l = 0), but there are three p orbitals (m l = −1,0,1), five d orbitals (m l = −2,−1,0,1,2), and seven f orbitals (m l = −3,−2,−1,0,1,2,3).

Filling up these orbitals with electrons builds atoms, and the way in which atoms are build up gives rise to the periodic table. 01.08.2011 · a crash course tutorial on atomic orbitals, quantum numbers and electron configurations + practice problems explained.cc academy videos are easy 101 crash co. At the first energy level, the only orbital available to electrons is the 1s orbital, but at the second level, as well as a 2s orbital, there are also orbitals called 2p orbitals.. Of the four, s and p orbitals are.

N shell subshells states electrons 1 k s. View all 118 elements in one interactive periodic table. If we are to discuss it in terms of orbitals that are occupied then we are only dealing with the 1s orbital. Explore each elements orbitals and electron configuration. 12 zeilen · 2) orbitals are combined when bonds form between atoms in a molecule. Of the four, s and p orbitals are. They offer a way to calculate the probability of finding an electron in a specified region around the nucleus of the atom. There are four different kinds of orbitals, denoted s, p, d and f each with a different shape. Of the four, s and p orbitals are.
